The Execution Stage of Technology Transfer
An Overview of the Execution Stage
The Execution stage is the heartbeat of pharmaceutical technology transfer, where the meticulous planning of the Readiness stage comes to fruition. This critical phase involves executing Process Performance Qualification (PPQ) and ensuring operational readiness and compliance at the Receiving Unit (RU). Here, we delve into the key roles and associated activities across various functions:
Key Roles and Activities
Business Function
In the Execution stage, the Marketing Authorisation Holder (MAH) Business function is actively engaged in crafting the launch strategy. This includes anticipating the completion of PPQ batches, preparing for regulatory submission, and collaborating closely with the Sourcing/Supply Chain function to align PPQ batches with estimated market demand. Multiple stage gates may be conducted in this phase, ensuring a thorough verification of readiness before crucial milestones.
Technology Transfer Project Manager
The TT PM plays a pivotal role in supporting the team during the Execution stage. Their focus is on removing any remaining obstacles for engineering and PPQ batches. This involves verifying all documentation is approved, compliance is met, and addressing change controls and deviations. The TT PM’s responsibility extends to guiding both the Sending Unit (SU) and RU teams in executing a person-in-plant program for seamless support and training. A key deliverable for the TT PM is approving the TT report and documenting the fulfillment of TT requirements.
Process Function
The RU Process function oversees qualification exercises, including shipping validation and PPQ runs. They investigate process-related deviations, support studies defined in the process VMP, and troubleshoot issues. During PPQ, the Process ensures that the knowledge gained during the Readiness stage is effectively incorporated into production batch records and procedures. Post PPQ, Process closes all deviations, gathers data, and issues validation reports.
Analytical Function
In the Execution stage, the Analytical function is fully operational, conducting testing on materials, in-process samples, finished products, and cleaning samples. The testing regimen for PPQ is typically more extensive than routine production. Additionally, Analytical oversees the packaging of stability samples as defined in the stability protocol.
Engineering Function
Having completed all qualification requirements, the Engineering function supports the Execution stage by addressing issues during engineering and PPQ runs. This includes investigating deviations and issuing change controls related to equipment or facilities. Engineering supports validation-related sampling, data analysis, and reporting to ensure all PPQ requirements are met.
Manufacturing Function
A major deliverable for Manufacturing in the Execution stage is the execution of engineering and PPQ batches. The SU team often participates during the engineering batch, contributing to process adjustments and improvements. The Manufacturing function provides critical feedback on operations, training effectiveness, and batch records. This feedback is incorporated into lessons learned before the handover to the RU post-transfer operations team.
Regulatory Function
The Regulatory function is actively involved in drafting the regulatory submission during the Execution stage. Frequent updates are provided to align submission timing and content with the finalized regulatory strategy. The Regulatory function also collaborates with Quality on Pre-Approval Inspection (PAI) preparation, ensuring alignment of claims made in the dossier, plant-level documentation, and storyboards.
Quality Function
Quality is integral during the Execution stage, releasing materials for use and ensuring product release compliance. The Quality function reviews and approves all documentation for PPQ, including protocols, reports, batch records, and deviation close-outs. It performs final inspection readiness activities in anticipation of the regulatory filing and may complete a mock inspection depending on the TT type.
Sourcing/Supply Chain Function
During the Execution stage, the Sourcing/Supply Chain function collaborates closely with the Business function to finalize shipping requirements. This function also works with Manufacturing and Engineering on PPQ requirements, including scheduling, material, and storage needs, establishing a clear supply chain and standards for production planning.
The Road to Successful Technology Transfer
The Execution stage is a multifaceted phase where various functions work collaboratively to execute PPQ and ensure all operational and compliance requirements are met. Collaboration, meticulous oversight, and effective communication across functions are paramount for successful execution, setting the stage for the subsequent Handover phase. The seamless execution of these activities is crucial for achieving excellence in pharmaceutical technology transfer. At this stage, when collaboration and communication are most critical, an experienced and skilled TT PM is essential to the successful and timely completion of the TT execution. This is why it is often best to rely on a strategic partner to provide skilled personnel if the RU and SU teams are overloaded or lack the requisite experience in delivering TT execution on time and on budget.
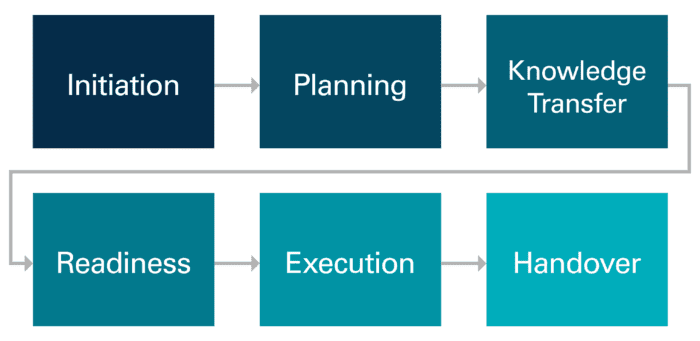
Follow CAI’s Tech Transfer blog series as we step through each of the stages of an effective technology transfer shown below.
Tags: Operational Readiness, Technology Transfer, execution, PPQ