Planning for a Talented Workforce: Building a Staffing Plan for New or Expanding GMP Facilities
Let’s contrast two very real scenarios:
- You are responsible for bringing a new GMP facility or line to full-scale production. Your board or shareholders expect you to have thought of everything that requires funding without excessive capital or operational budget overrun. Based on your experience at a previous facility, you estimate you will need X number of operators, Y number of technicians, and Z number of analysts, scientists, and engineers. 12 months later, you recognize that you were off by a factor of 2! You return to the board and ask for expanded resources, and they challenge you to produce the evidence from both your previous and revised estimate. Explaining that your previous method was essentially an educated guess is an uncomfortable position, to say the least. This scenario is played out time and again as sites move from capital projects to operations.
- Now imagine you have a “map” of the processes, tasks, and production capacity needed during full-scale site operations…
…and you have used this to define the roles needed in manufacturing and across the other site departments.
Not only is your conversation with the board more productive and accurate, but you also have the makings of a full production schedule, accelerating your transition from capital project mode to operations mode.
Many organizations begin their talent planning by recalling the staffing levels of a previous operation — simply estimating the number of people needed in each “department” based on loosely related history. While this approach might seem sufficient, it places a heavy burden upon your department leaders to predict their needs without the benefit of their operationally experienced team members who know the processes best. Even then, there is the risk of falling short in defining your resource needs for two primary reasons: 1) without mapping out ALL processes that support production (including deviation investigations, change controls, and associated CAPAs and continuous improvement efforts), it becomes evident that the demands of the projected staff roles will stretch the bandwidth of even the most dedicated and competent team members, and 2) evolving therapies, processes, systems, and digitization are changing the roles and staffing requirements at a rapid pace.
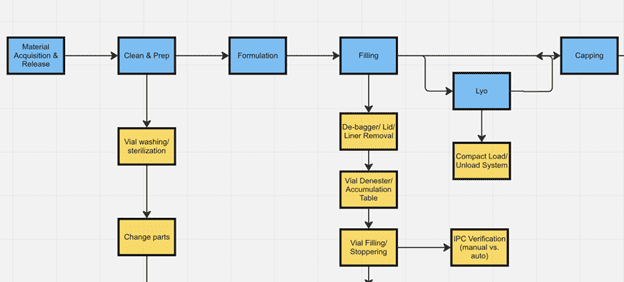
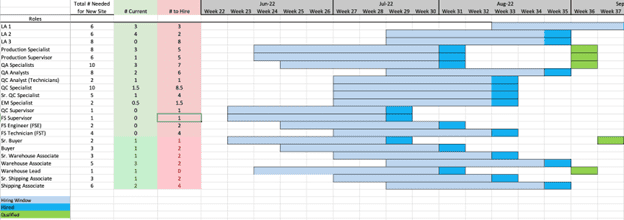
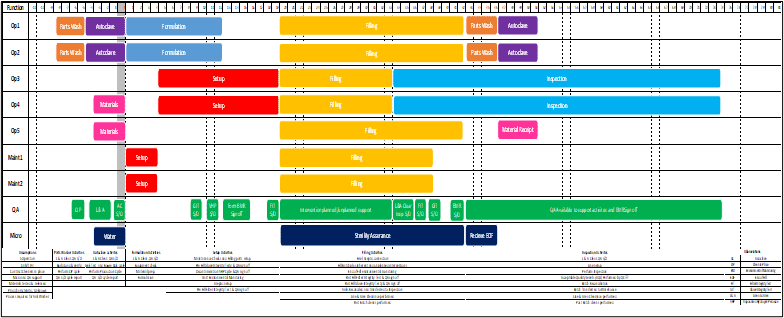
The best approach is a methodical analysis that starts by listing, then mapping, every system and process involved in performing the tasks within and in support of the site. This enables the next step, which is assigning specific roles to each process task. By mapping the cross-functional process flows hour-by-hour (or at least day-by-day during early planning or for lower volume sites), it becomes clear how many of each role (designating specialty and level of seniority) is required to execute production for a set period, a given campaign duration, or set of batches. Combining the data from this approach with the site capacity plan will yield a shift-based production and support team schedule, along with the final staffing plan for use in budget and workforce acquisition planning.
Data-driven analysis based on current best practices, actual plant design, and stakeholder input may take more time than copying what was done last time. However, the upfront investment may have one of the highest ROIs for any action you take during startup.
Tags: GMP, HP, human performance, Workforce, facilities, staffing